Our laboratory investigates the interactions between stromal and cancer cells in the tumour microenvironment
LivTME CANCER RESEARCH TEAM
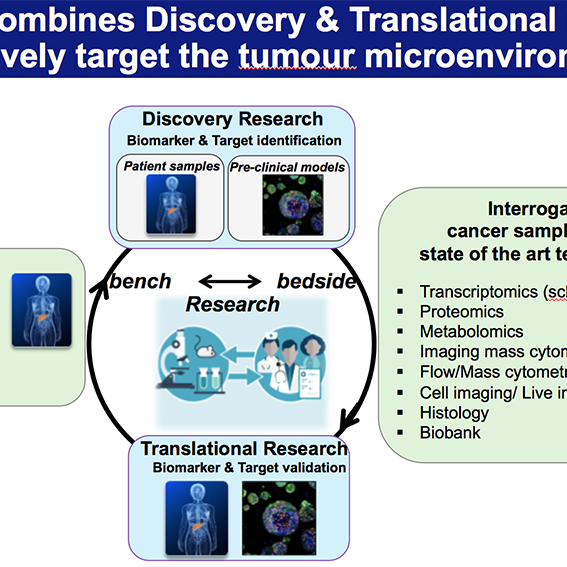
We utilise a variety of approaches such as primary and metastatic pre-clinical tumour models, cell biology, bioinformatics, and validation of findings in clinical samples.
LivTME
“Understanding which immune cell populations or functions are supporting tumour growth and metastasis…to stop cancer.”
Prof Ainhoa Mielgo
Our Research Goals
LivTME CANCER RESEARCH TEAM
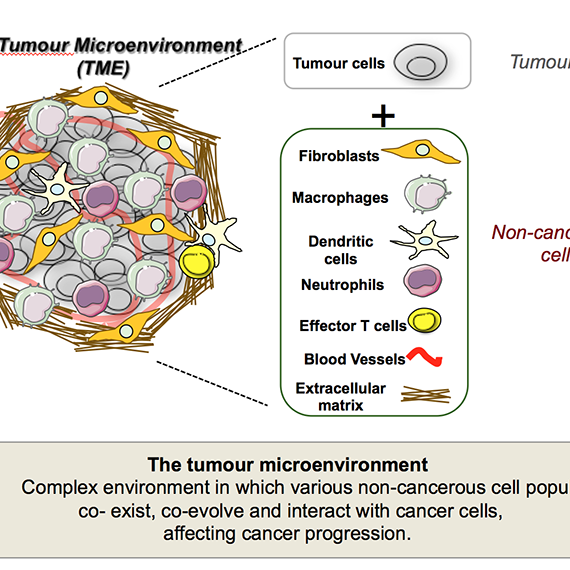
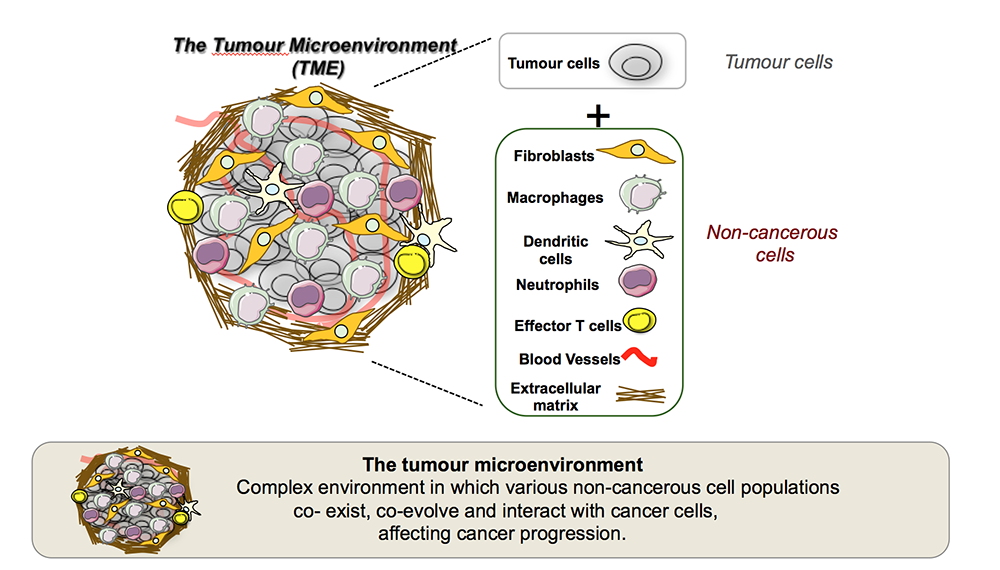
Tumours are not composed solely of cancer cells. Instead, they are complex ecosystems that include a wide variety of non-cancerous cells, such as immune cells, fibroblasts, and vascular cells, that together form the tumour microenvironment. These cells coexist, co-evolve, and interact dynamically with cancer cells, profoundly influencing tumour growth, metastasis, and responses to therapy.
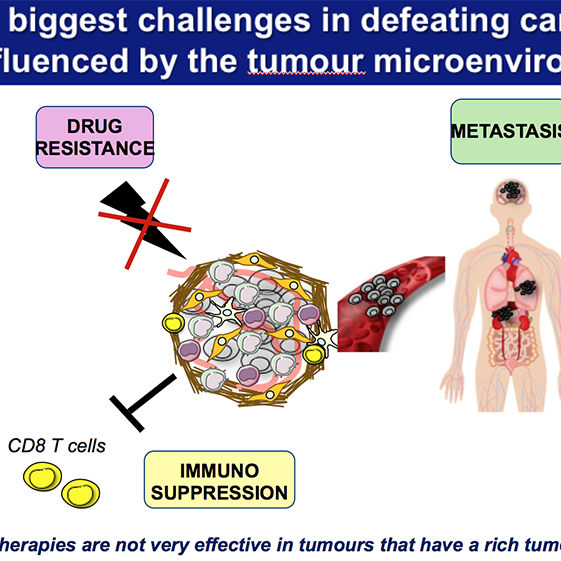
While localized tumours can often be surgically removed with curative intent, the prognosis worsens significantly once cancer spreads to distant organs – a process known as metastasis. In pancreatic cancer, the liver is a predominant site of metastasis, where the tumour microenvironment becomes particularly immunosuppressive and resistant to current treatments.
Our previous work has shown that tumour-associated macrophages are key drivers of both metastasis and immune evasion, particularly in the liver, where they help create a permissive niche that shields cancer cells from immune attack. However, these macrophages are highly plastic and can be reprogrammed to support anti-tumour immunity.
The central goal of our lab is to understand how immune cells and other stroma cells interact with cancer cells and how these interactions shape tumour progression, metastatic colonization, and resistance to therapy. We are particularly interested in the mechanisms of immune evasion in liver metastasis and aim to identify specific immune cell populations and functions that support this process. Ultimately, our research seeks to develop novel diagnostic markers and therapeutic strategies to overcome immune suppression and prevent or treat metastatic pancreatic cancer
LivTME
“Our research aims to uncover the complex role of the tumour microenvironment in driving pancreatic cancer liver metastasis, with a focus on immune evasion and stromal interactions that facilitate metastatic progression and resistance to therapy.”
Prof Michael Schmid
Understanding the role of stromalpartners at the metastatic site
RESEARCH
The Metastatic Niche
A MULTISTAGE PROCESS
Metastasis is a critical characteristic of pancreatic cancer and the primary cause of mortality in affected individuals. The spread of cancer to distant sites occurs in multiple stages, beginning with the detachment of cancer cells from the primary tumour and culminating in the development of detectable macro-metastases in distant organs.
Although tumours can release a large number of cancer cells into the bloodstream, only a tiny fraction of these cells are able to successfully establish a new tumour at a distant site. Consequently, the ability of these disseminated tumour cells to colonize a metastatic site is a crucial and rate-limiting step in metastasis. Identifying the factors that influence this step presents potential targets for therapeutic intervention.
While tumour cells are the main drivers of metastasis, recent research indicates that immune cells – particularly specific immune cells – play a vital role in promoting metastatic spread.
In fact, primary tumours can trigger the recruitment of circulating immune cells to the metastatic site, where they contribute to the creation of a metastatic niche that supports cancer cell survival and growth.
Macrophages in Pancreatic CancerLiver Metastasis
UNDERSTANDING MACROPHAGE SUBSETS
Macrophages are key components of the tumour microenvironment and play a pivotal role in the progression and metastasis of pancreatic cancer, particularly to the liver. In the metastatic niche, tumour-associated macrophages (TAMs) are recruited and educated by cancer cells to adopt an immunosuppressive, pro-tumour phenotype. These macrophages facilitate several steps of the metastatic process: they promote cancer cell survival in the circulation, support extravasation into the liver tissue, and help establish a permissive microenvironment that enables metastatic colonization.
In the liver, TAMs interact with both resident immune cells and stromal components to suppress anti-tumour immune responses and enhance tissue remodelling and immune evasion. Their ability to dampen cytotoxic T cell activity and promote tolerance is a key barrier to effective immunotherapy in metastatic pancreatic cancer.
However, their plasticity also presents a therapeutic opportunity- reprogramming TAMs to adopt a pro-inflammatory, anti-tumour phenotype could restore immune surveillance and inhibit metastatic progression.
Understanding the distinct roles and origins of macrophage subsets in the liver metastatic niche is critical for developing targeted therapies that disrupt their tumour-supportive functions without compromising host immunity.
618
The cancer incidence rate in NW England is 618.2 per 100,00
3%
Pancreatic cancer is 3% of all new cancer cases in males and females
8%
Estimated percentage of deaths of pancreatic cancer patients
Our Research Focus
TOWARDS TRANSFORMATIVE TREATMENTS
Our research is dedicated to unravelling how the pancreatic cancer tumour microenvironment (TME) promotes immune evasion and metastatic colonization, with a specific focus on the liver. We are particularly interested in how immune cells are reprogrammed within the TME and metastatic niche to support tumour progression and resistance to therapy. Understanding these cellular interactions is critical for developing strategies to restore anti-tumour immunity and prevent metastatic spread.
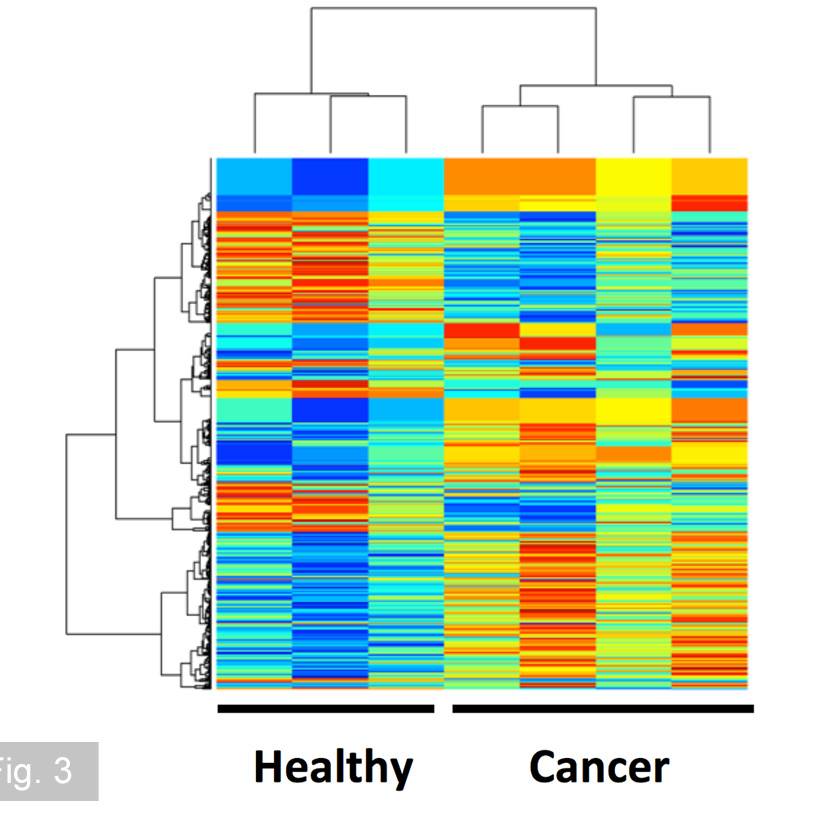
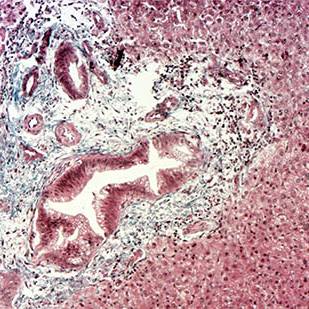
A central strength of our research program is our close and ongoing collaboration with clinical colleagues, including pancreatic surgeons, medical oncologists, and pathologists. These partnerships enable us to obtain high-quality, well-annotated patient-derived tissue samples- including primary tumours, liver metastases, and matched adjacent non-tumour tissue – which are essential for translating our findings into clinically relevant insights. Working closely with surgeons, we access resected specimens at the time of surgery, while collaboration with oncologists allows us to study tissues from patients receiving neoadjuvant or adjuvant therapy. Through pathologist-led evaluation, we ensure accurate tissue characterization, spatial mapping, and correlation with clinical outcomes.
To complement patient studies, we employ a suite of pre-clinical models, to dissect the timing, function, and therapeutic vulnerability of immune populations.
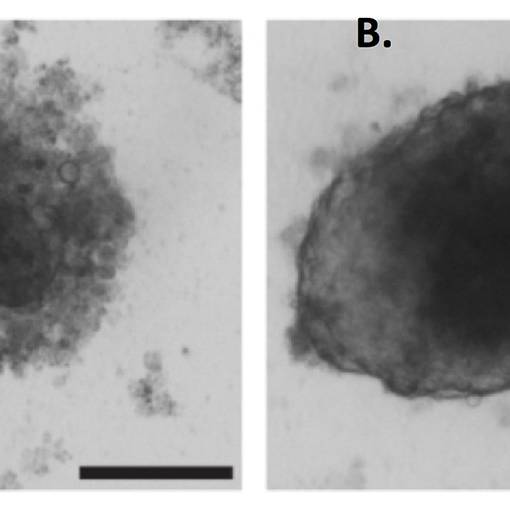
We also leverage advanced 3D in vitro culture systems, such as patient-derived organoids and organotypic co-cultures with immune cells, to model specific aspects of immune–tumour interaction in a controlled setting. These systems provide a platform for mechanistic studies and drug testing, bridging the gap between discovery and clinical application.
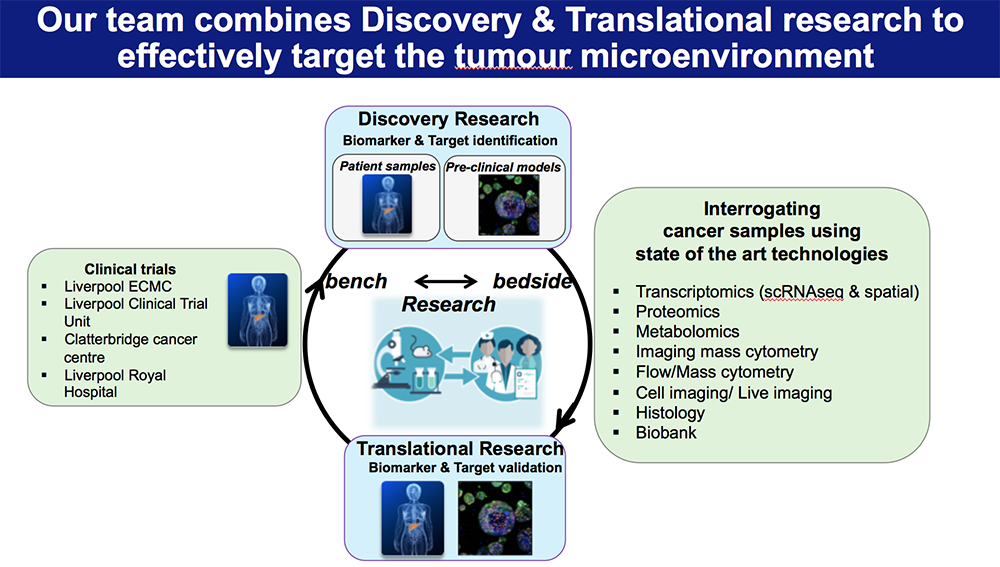
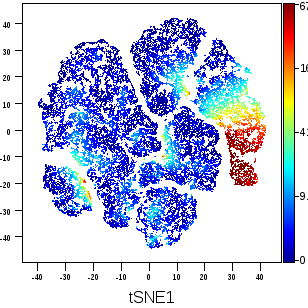
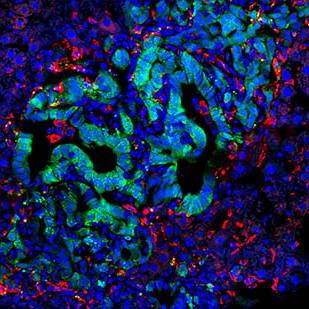
Our research is powered by state-of-the-art technologies, including single-cell RNA sequencing, spatial transcriptomics, multiplex immunofluorescence imaging, and high-dimensional flow cytometry. These tools allow us to map the cellular architecture of the TME and metastatic niche at high resolution, identify immune escape mechanisms, and uncover actionable therapeutic targets.
By integrating basic science, translational research, and clinical collaboration, our work aims to illuminate the immune mechanisms that underlie pancreatic cancer metastasis and resistance. Ultimately, we seek to inform the development of new diagnostics and immune-based therapies tailored to the biology of each patient’s tumour.